Right now, children 5-11 years old are eligible for smaller dosage vaccines.
They come exclusively from Pfizer BioNtech.
That same company is looking to apply to the United States Food & Drug Administration for approval for a younger age group.
Pfizer has been conducting studies of their kids vaccine for children 6 months to 5 years old.
If approved, the vaccine could be readily available to US residents by March.
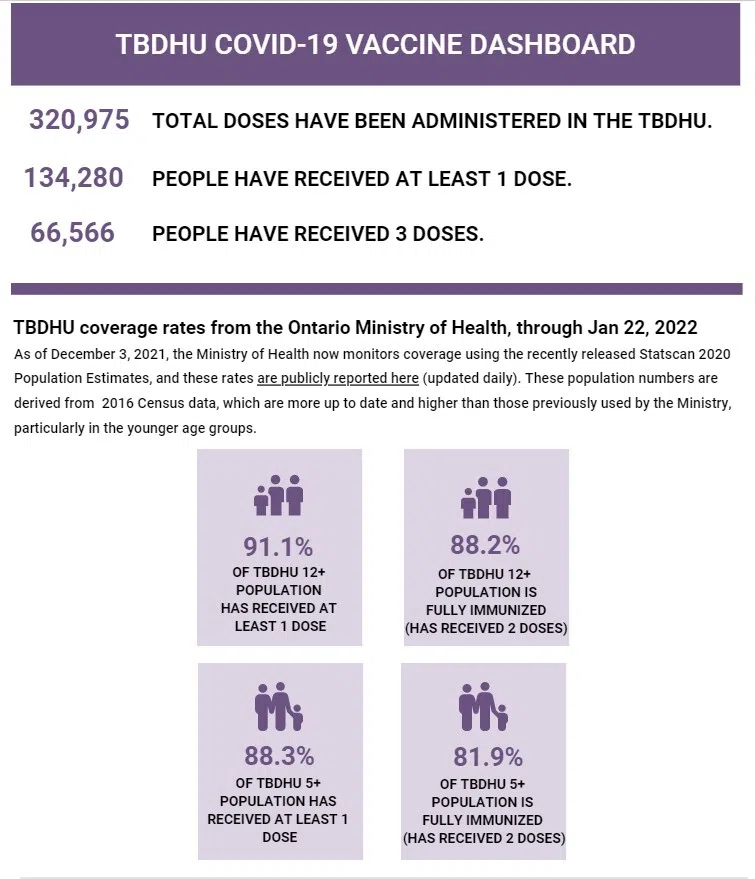
The Center for Disease Control now considers three doses to be “up to date” for those 12 and up.
Locally, just over 60 per cent of people have three doses of the vaccine. 56 per cent of kids 5-11 have one, and only eight per cent have had two doses.